醫(yī)學(xué)/安全事務(wù)部由具有豐富的臨床醫(yī)學(xué)背景和臨床試驗(yàn)經(jīng)驗(yàn)的國內(nèi)外專業(yè)人士組成。 依托多年開展醫(yī)療器械臨床試驗(yàn)及注冊(cè)的經(jīng)驗(yàn),基于對(duì)國家相關(guān)法律法規(guī)的熟讀與 理解,能夠進(jìn)行臨床試驗(yàn)方案設(shè)計(jì)、臨床試驗(yàn)可行性分析及咨詢、臨床試驗(yàn)報(bào)告撰 寫、醫(yī)療器械臨床評(píng)價(jià)報(bào)告撰寫、醫(yī)學(xué)信息檢索、醫(yī)學(xué)翻譯等內(nèi)容。作為中立第 三方服務(wù)機(jī)構(gòu),可提供專業(yè)的臨床試驗(yàn)安全管理服務(wù),包括臨床終點(diǎn)事件評(píng)審委員 會(huì)(CEC)、數(shù)據(jù)安全監(jiān)管委員會(huì)(DSMB)、AE/SAE事件管理、臨床實(shí)踐判定 的管理與協(xié)調(diào)。 依托“醫(yī)心”公眾號(hào)開展醫(yī)學(xué)信息傳播服務(wù),包括醫(yī)學(xué)專業(yè)學(xué)術(shù)推廣的策劃、創(chuàng)意、實(shí) 施;為客戶打造“個(gè)體化”設(shè)計(jì)、制作的專屬醫(yī)學(xué)信息推廣平臺(tái)。
Medical Affairs
The Department of Medical Affairs consists of domestic and foreign professionals with rich clinical medicine background and clinical trial experience.
Based on years of experience in carrying out clinical trials and registration of medical devices, the familiar reading and understanding of relevant national laws and regulations, clinical trial design, clinical trial feasibility analysis and consultation, clinical trial report writing, medical device clinical evaluation report writing, medical information retrieval, medical translation and other content can be conducted.
As a neutral third party service organization, professional clinical trial safety management services, including clinical endpoint event review committee (CEC), data and safety monitoring board (DSMB), AE/SAE event management, and clinical practice judgment management and coordination can be provided.
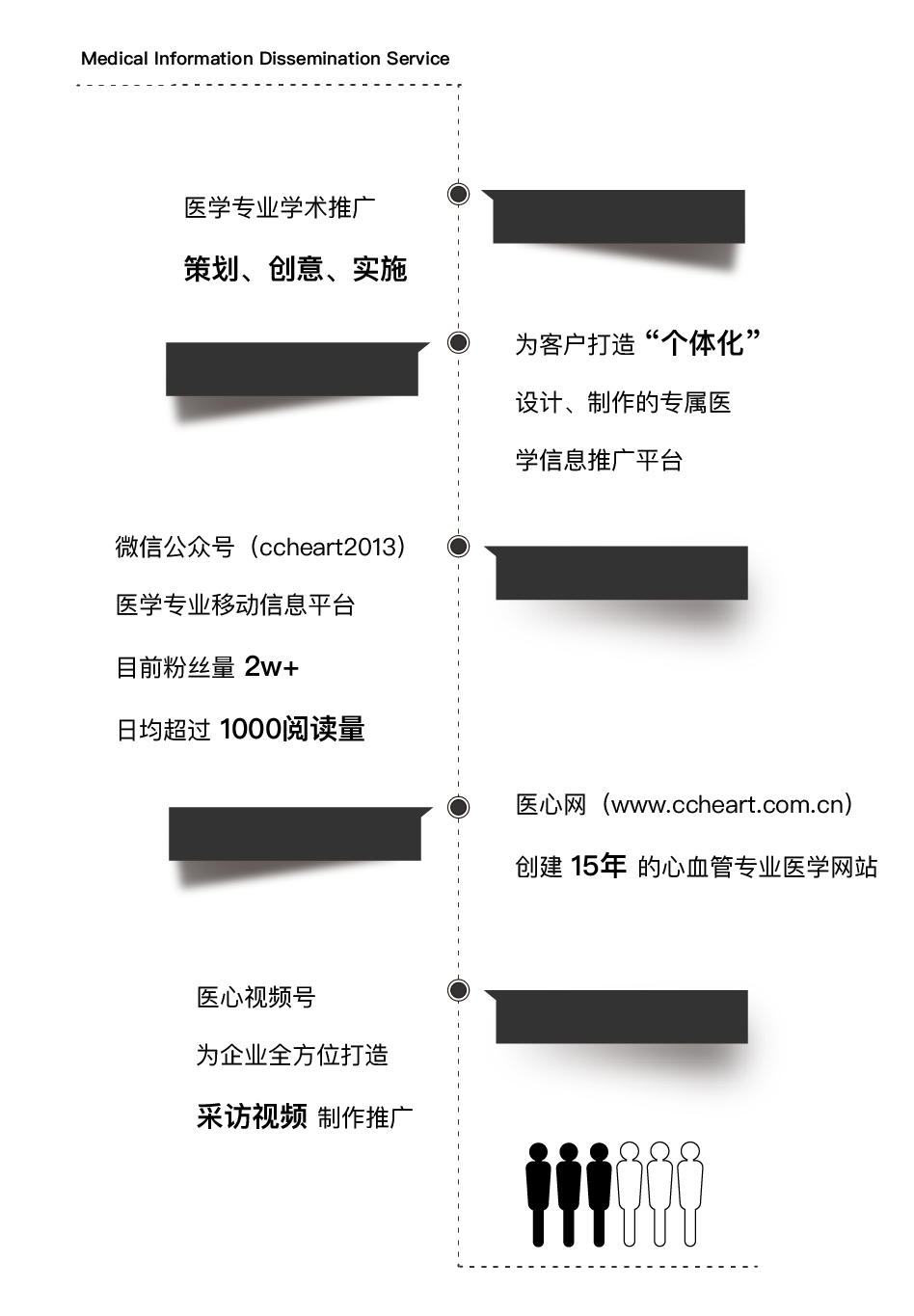
Relying on the "Yixin" official account, medical information dissemination services, including the planning, creativity and implementation of medical academic promotion can be carried out; moreover, exclusive medical information promotion platform for customers with "individualized" design and production can be designed.
醫(yī)學(xué)專業(yè)服務(wù)
醫(yī)學(xué)信息傳播服務(wù)
安全管理
永銘醫(yī)學(xué)可提供專業(yè)的臨床試驗(yàn)安全管理服務(wù),包括臨床終點(diǎn)事件評(píng)審委員會(huì)(CEC)、數(shù)據(jù)安全監(jiān)管委員會(huì)(DSMB)、AE/SAE事件管理、臨床實(shí)踐判定的管理與協(xié)調(diào)。
安全管理團(tuán)隊(duì)成員均具有臨床醫(yī)學(xué)背景及心血管臨床試驗(yàn)相關(guān)經(jīng)驗(yàn),沿用高標(biāo)準(zhǔn)的專業(yè)的SOP,確保臨床終點(diǎn)事件的評(píng)審結(jié)果客觀準(zhǔn)確。
至今共評(píng)審了國內(nèi)外上市前及上市后的多個(gè)個(gè)心血管相關(guān)的臨床研究項(xiàng)目,完成2,000例終點(diǎn)事件CEC的評(píng)審。完成了超過5,000例的AE/SAE的事件管理。完成了多個(gè)臨床研究項(xiàng)目的數(shù)據(jù)安全監(jiān)管委員會(huì)(DSMB)的支持工作。


